|
|
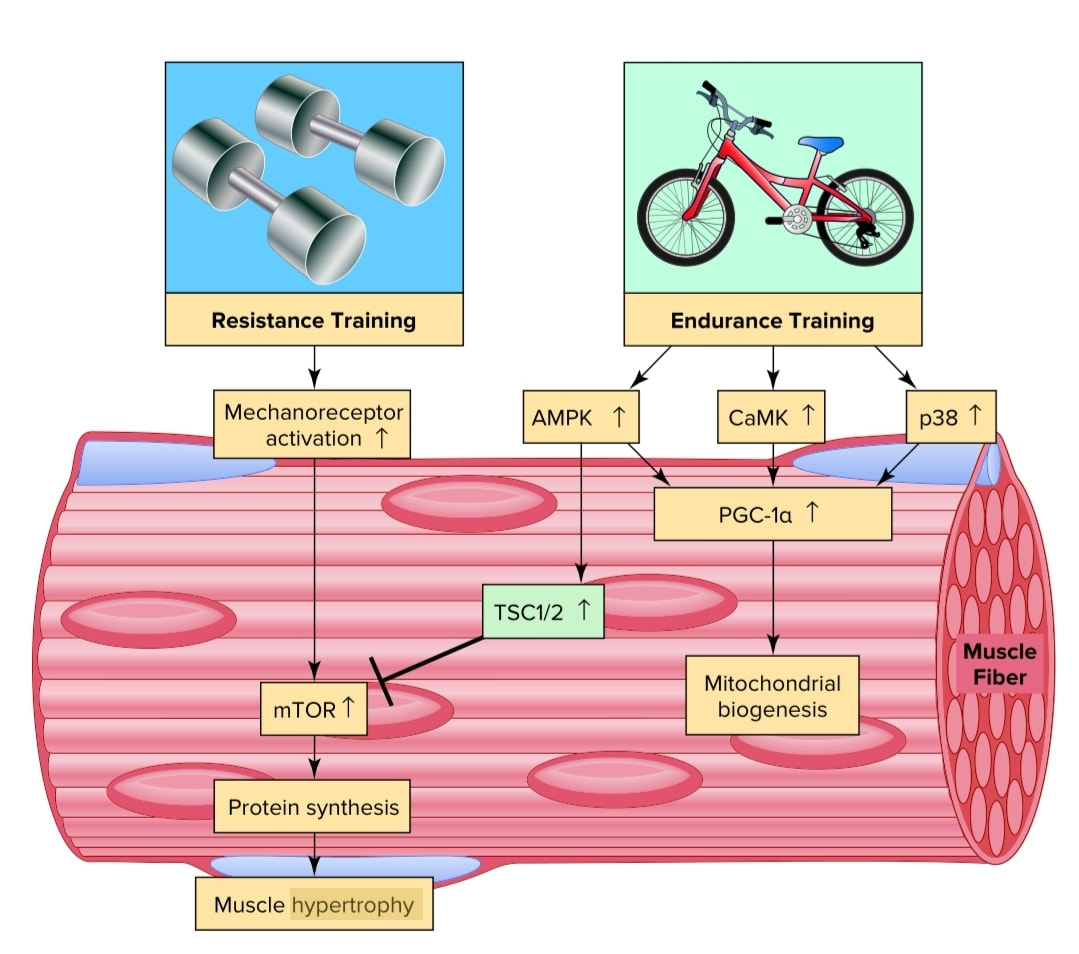
Intracellular signaling networks mediating exercise-induced skeletal muscle responses to resistance and endurance exercise training programs. Resistance exercise training activates the mTOR pathway that promotes pro-tein synthesis by increasing translation. Endurance exercise training activates several secondary signals to promote an increase in PGC-1α and increased mitochondrial biogenesis. Note that activation of AMPK by endurance training can inhibit mTOR signaling via the tuberous sclerosis complex 2 (TSC 2) and suppress resistance training-induced protein synthesis. The interaction between these two intracellular signaling networks can potentially explain why concurrent resistance and endurance training programs can reduce strength gains observed with resistance training alone.
Mechanoreceptors
Skeletal muscle contains several types of sensory receptors. These include muscle spindles and Golgi tendon organs. Collectively, these sensory receptors are often called muscle mechanoreceptors because these receptors are sensitive to mechanical (pressure/length) changes in the muscle. Importantly, these mechanoreceptors not only send information to higher brain centers about movement patterns but these receptors also send afferent neural information to both the cardiovascular and respiratory control centers; this information is used to regulate the car-diovascular and respiratory response to exercise.
mTOR is a protein kinase that is the major regulator of protein synthesis and muscle size. Activation of mTOR promotes increased translation that results in increased protein synthesis. Specifically, mTOR is a secondary signaling molecule in muscle adaptation to resistance exercise training that is activated by stimulation of a mechanoreceptor on the sarcolemma of the muscle.
CaMK (Calmodulin-Dependent Kinase) Calmodulin- dependent kinase is activated during endurance exercise training. This vital kinase exerts influence on exercise induced muscle adaptation by contributing to the activation of PGC-1α. The primary upstream signal to activate CaMK is increased cytosolic calcium levels.
P38 Mitogen activated kinase p38 (p38) is an important signaling molecule that is activated in muscle fibers during endurance exercise. Although several cellular stresses can activate p38, it seems likely that exercise induced production of free radicals is a major signal to activate p38 in muscle fibers. Once activated, p38 can contribute to mitochondrial biogenesis by activating PGC-1α.
AMPK is an important signaling molecule that is activated during both high intensity interval training, and submaximal endurance
exercise training due to changes in muscle fiber phosphate/energy levels. This key molecule regulates numerous energy producing pathways in muscle by stimulating glucose uptake and fatty acid oxidation during exercise.
AMPK is also linked to the control of muscle gene expression by activating transcription factors associated with fatty acid oxidation and mitochondrial biogenesis.
PGC-1α This key molecule is activated by both high-intensity interval training and submaximal endurance exercise and is considered the master regulator of mitochondrial biogenesis in cells. Indeed, PGC-1α assists transcriptional activators that promote mitochondrial biogenesis in skeletal muscle following endurance training. Furthermore, PGC-1α regulates many other endurance exercise-mediated changes in skeletal muscle, including the formation of new capillaries (angiogenesis), a fast-to-slow muscle fiber type shift, and synthesis of antioxidant enzymes. PGC-1α also plays a role in exercise-induced increases in muscle’s ability to metabolize fat and to take up glucose into the muscle fiber. Several factors activate PGC-1α during exercise, including AMPK and p38.
Skeletal muscle contains several types of sensory receptors. These include muscle spindles and Golgi tendon organs. Collectively, these sensory receptors are often called muscle mechanoreceptors because these receptors are sensitive to mechanical (pressure/length) changes in the muscle. Importantly, these mechanoreceptors not only send information to higher brain centers about movement patterns but these receptors also send afferent neural information to both the cardiovascular and respiratory control centers; this information is used to regulate the car-diovascular and respiratory response to exercise.
mTOR is a protein kinase that is the major regulator of protein synthesis and muscle size. Activation of mTOR promotes increased translation that results in increased protein synthesis. Specifically, mTOR is a secondary signaling molecule in muscle adaptation to resistance exercise training that is activated by stimulation of a mechanoreceptor on the sarcolemma of the muscle.
CaMK (Calmodulin-Dependent Kinase) Calmodulin- dependent kinase is activated during endurance exercise training. This vital kinase exerts influence on exercise induced muscle adaptation by contributing to the activation of PGC-1α. The primary upstream signal to activate CaMK is increased cytosolic calcium levels.
P38 Mitogen activated kinase p38 (p38) is an important signaling molecule that is activated in muscle fibers during endurance exercise. Although several cellular stresses can activate p38, it seems likely that exercise induced production of free radicals is a major signal to activate p38 in muscle fibers. Once activated, p38 can contribute to mitochondrial biogenesis by activating PGC-1α.
AMPK is an important signaling molecule that is activated during both high intensity interval training, and submaximal endurance
exercise training due to changes in muscle fiber phosphate/energy levels. This key molecule regulates numerous energy producing pathways in muscle by stimulating glucose uptake and fatty acid oxidation during exercise.
AMPK is also linked to the control of muscle gene expression by activating transcription factors associated with fatty acid oxidation and mitochondrial biogenesis.
PGC-1α This key molecule is activated by both high-intensity interval training and submaximal endurance exercise and is considered the master regulator of mitochondrial biogenesis in cells. Indeed, PGC-1α assists transcriptional activators that promote mitochondrial biogenesis in skeletal muscle following endurance training. Furthermore, PGC-1α regulates many other endurance exercise-mediated changes in skeletal muscle, including the formation of new capillaries (angiogenesis), a fast-to-slow muscle fiber type shift, and synthesis of antioxidant enzymes. PGC-1α also plays a role in exercise-induced increases in muscle’s ability to metabolize fat and to take up glucose into the muscle fiber. Several factors activate PGC-1α during exercise, including AMPK and p38.